Joseph Priestley
1733-1804
Priestley was born on March 13th, 1773 in West Yorkshire, England. His early research mostly focused on electrical science. His book, The History and Present State of Electricity, was very popular then. In 1767, Priestley began to systematically study various gases, and established himself as one of the experts in the field. Priestley discovered many new gases, in which the most famous one was oxygen he produced through heating mercury oxide with a burning glass. Because he was a firm believer of phlogiston theory, he named oxygen “dephlogisticated gas”. Priestley was a very industrious researcher and often worked on the desk until he could no longer hold the pen. Because his political standing was against the English government, he was forced to move to the United States in 1794. Priestley died on February 6th, 1804 (aged 70) in Pennsylvania, the US. His main contributions to science are:
Invented soda water (water with carbon dioxide).
Discovered oxygen.
Developed systematic experiments to generate and study many gases, discovered several new gases including nitrogen monoxide and hydrogen chloride.
Discovered that plants could absorb carbon dioxide and release oxygen.
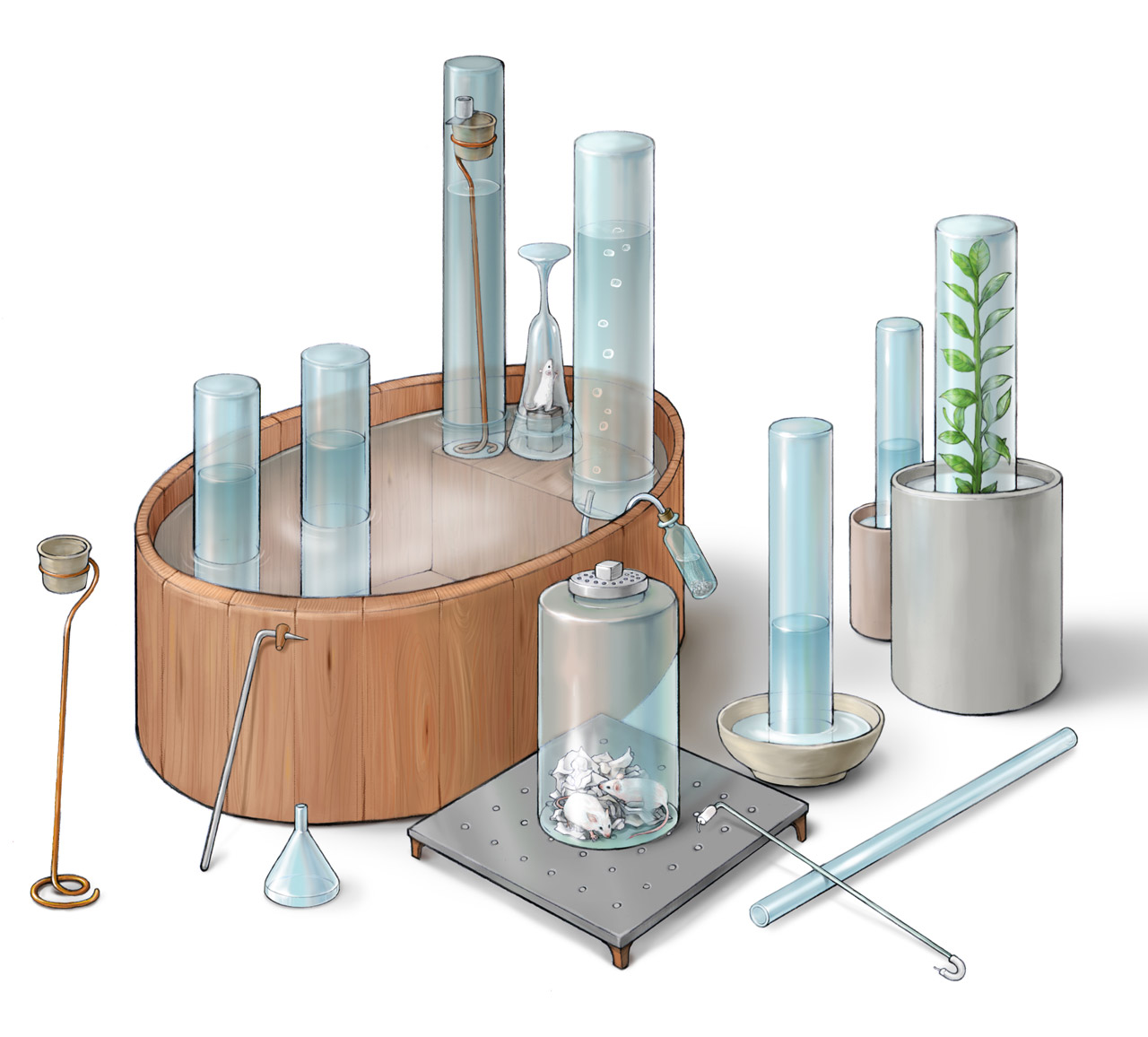
Priestley's Instruments
Above is Priestley’s various apparatuses for conducting gas experiments described in his Experiments and Observations on Different Kinds of Air published in 1774 (a photorealistic CG reconstruction can be found here). The figure was made based on the original illustration in the frontispiece of his book. The trough with a platform on one side was essential to his experiments: collecting gases, transferring gases, and other experiments were all performed inside this trough. Priestley prepared many gases, including nitric oxide, nitrogen dioxide, carbon monoxide, carbon dioxide, nitrogen, oxygen, hydrogen, hydrogen chloride, ammonia, sulfur dioxide, some of which were first discovered by him, such as oxygen. For gases that dissolved in water, he used mercury to collect them. Priestley carefully studied the physical and chemical properties of many gases. He also discovered that plants could absorb carbon dioxide and release oxygen. However, he did not study the function of light in this process.
Above is Priestley’s various apparatuses for conducing gas experiments described in his Experiments and Observations on Different Kinds of Air published in 1774 (a photorealistic reconstruction of the apparatus utilizing electricity can be found here). The figure was made based on the original illustration in the tailpiece of his book. Priestley improved Hales apparatus for preparing gas at high temperature. Through ingenious design, he studied reactions of gases under electrical spark. Priestley was one of the first scientists who discovered oxygen. In 1774, he prepared oxygen by heating mercury oxide with a burning glass. He found that oxygen did not dissolve in water and it made combustion stronger. Priestley was a firm believer of phlogiston theory. Based on this theory, combustion was a process in which phlogiston was released from the flammable substances. He proposed that the intense combustion inside oxygen was due to oxygen contained no or very little phlogiston and it easily absorb phlogiston from other susbstances. As a result, he called oxygen “dephlogisticated air” and nitrogen, which did not support combustion, “phlogisticated air”.
Other Chemists