Henry Cavendish
1731-1810
Henry Cavendish was born on October 10th, 1731 in Nice, Kingdom of Sardinia. Although he was bone in a wealthy family, he was seldom engaged in any social activities and devoted himself entirely to scientific research. Cavendish’s research was very broad and productive. However because he never published results which he was not completely satisfied, he only published less than 20 research papers in his lifetime. In his unpublished manuscripts, many results were very advanced during his time. Cavendish was famous for his accuracy in his experiments. The density of Earth which Cavendish published in a 1789 paper is different from current value by only 1%. Cavendish died on February 24th, 1810 (aged 78) in London, England. His main scientific contributions are:
In Chemistry, comprehensively investigated the properties of hydrogen gas, collected water-soluble gases with mercury for the first time, accurately determined the ratio of hydrogen and oxygen in water, accurately determined the composition of the air.
In electrical science, demonstrated that electric force was inversely proportional to the distance between two charges, distinguished the quantity of electric charge and electric potential.
In thermal science, used the theory of latent heat to accurately determined the melting point of mercury, sulfuric acid, nitric acid and other liquids.
In Earth physics, determined the density of Earth through rigorous experiment.
Cavendish's Instruments
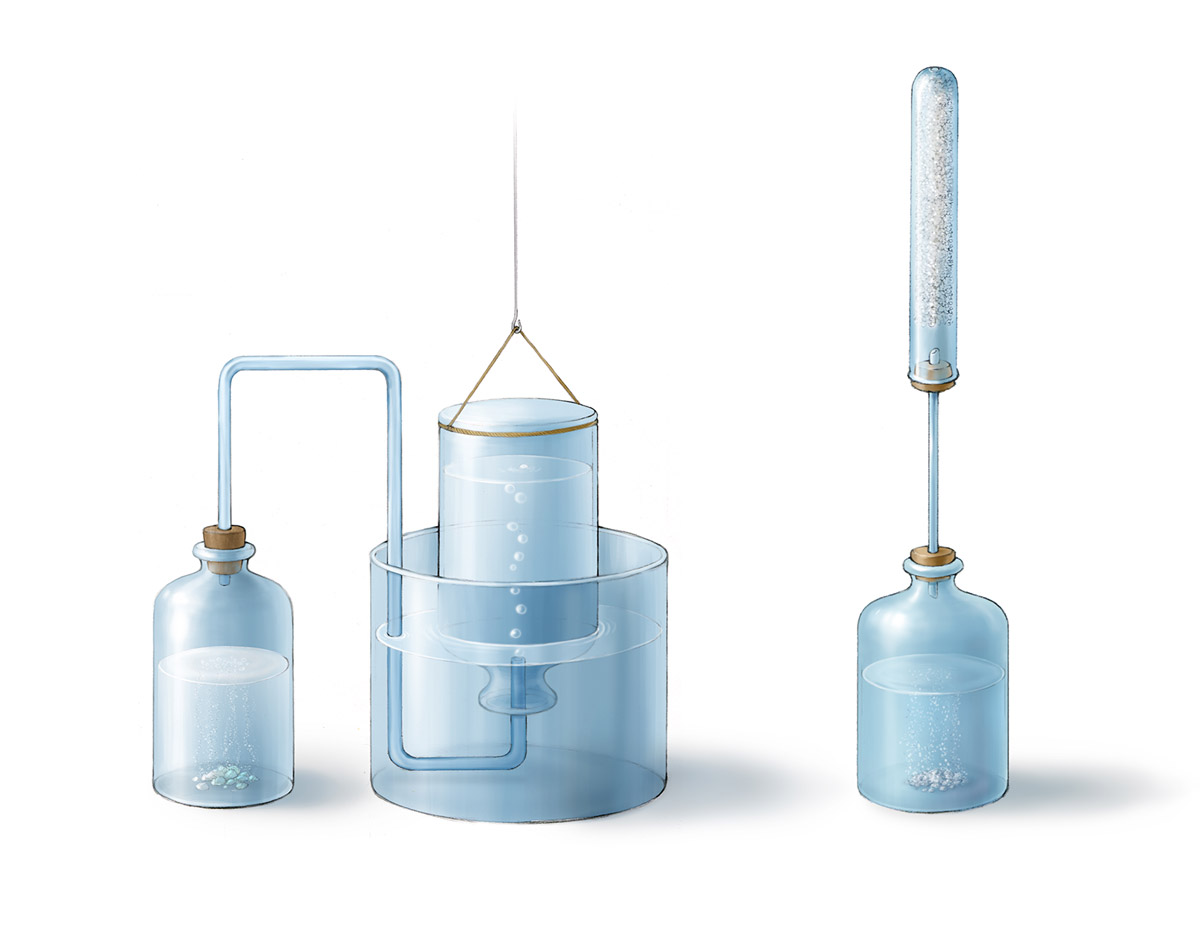
Above are Cavendish’s apparatuses for hydrogen gas preparation and weight measurement described in his paper On Factitious Airs published in 1766. Cavendish found that when metals such as zinc and iron were mixed with hydrochloric acid or dilute sulfuric acid, a flammable gas was released. He called this gas “inflammable air”. At that time, Cavendish was a believer of phlogiston theory. Since when the same amount of a certain metal reacted with different acids resulting in the same amount of inflammable air, he argued that this air came from the metal and it was the phlogiston of the metal. We now know that this is not true since inflammable air, i.e. hydrogen gas, came from hydrogen ions of the acid. In addition, Cavendish determined the relatively accurate density of hydrogen used the apparatus on the right. Inside the tube were powders of potassium carbonate which adsorbed the moisture mixed in the hydrogen gas. Through measuring the change of weight before and after the reaction, the weight and density of hydrogen could be determined.
Above is Cavendish’s apparatus for transferring and mixing gases described in his paper On Factitious Airs published in 1766 (a photorealistic CG reconstruction of an improved apparatus can be found here). Cavendish knew that mixture of hydrogen and air exploded upon ignition. With this apparatus, he prepared hydrogen-air mixture with different proportion. He discovered the strongest exploration occurred when the hydrogen-air ratio was 3:7, corresponding to hydrogen-oxygen ratio of 2.04:1. This value is very close to the 2:1 ratio of hydrogen and oxygen in water. At the time of the experiment, Cavendish did not know the existence of oxygen. Later after the discovery of oxygen, Cavendish used an improved apparatus to study the explosion of hydrogen-oxygen mixture and determined the hydrogen-oxygen ratio inside water was 2.02:1.
Other Chemists