Antoine Lavoisier
1743-1794
Lavoisier was born on August 26th, 1743 in Paris, France. Lavoisier discovered no new substances nor new chemical reactions, but his greatest achievement was the establishment of a new theoretical framework within which others’ experimental results could be properly explained. Lavoisier stressed on the importance of accurate measurements, and used quantitative experiments to test his ideas. He started a chemical revolution which put an end to the once popular phlogiston theory. In the center of this revolution was the elucidation of oxygen’s function in combustion. During French Revolution, Lavoisier, as a nobleman, was accused of several crimes, and was eventually guillotined at age of 50 on May 8th, 1794 in Paris, France. Lavoisier’s main contributions to science are:
Through quantitative experiments, elucidated the role of oxygen in combustion and metal calcination, putting an end to phlogiston theory.
Presented modern concepts of chemical elements, invalidated the Greek four element theory.
Invented modern nomenclature for chemical substances to replace old empirical one.
Authored the famous textbook Traité Élémentaire de Chimie, popularized the new chemical system.
Lavoisier's Instruments
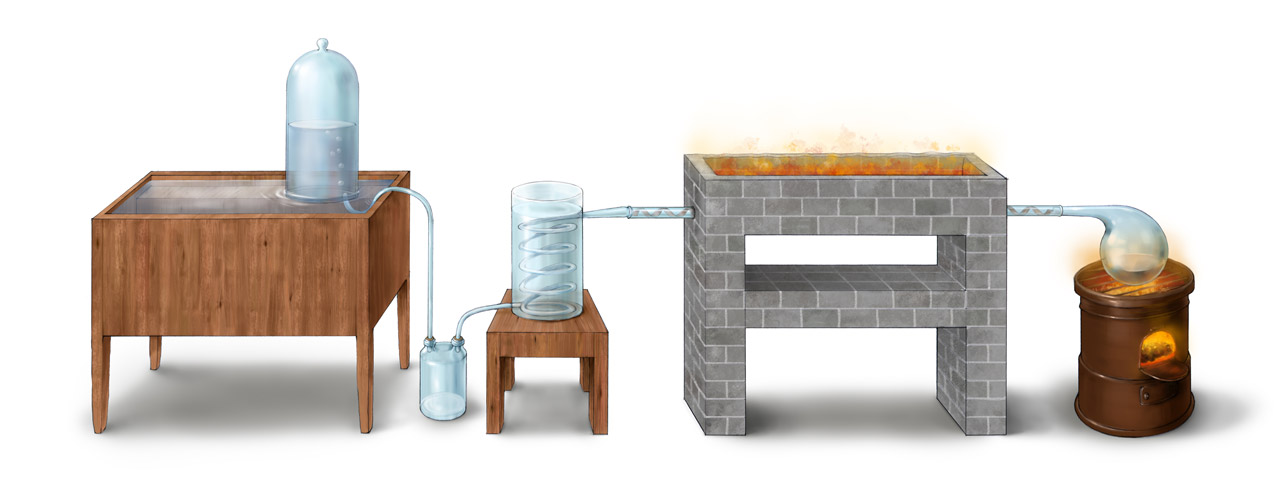
Above shows Lavoisier’s apparatus for studying mercury oxidation in closed environment described in his Traité Élémentaire de Chimie published in 1789 (a photorealistic CG reconstruction can be found here). The system contained mercury in the resort and normal air sealed by a bell jar placed in the mercury reservoir. After heating the mercury in the resort for several days, red mercury oxide was observed on mercury surface. The mercury level inside the bell jar rose up because the consumption of oxygen. When the amount of mercury oxide no longer increased, the heating was terminated and the amount of gas volume decrease was measured. Lavoisier found that the gas loss was 16% of the total volume. The the mercury oxide was removed and heated again, the volume of oxygen generated was measured. It was found that the volume was the same as the 16% volume loss. The oxygen percentage (16%) was not accurate, which could be due to not all oxygen react with mercury. From this experiment, we can feel Lavoisier’s emphasize on the conservation of mass in his experiment design.
Above is Lavoisier’s apparatus for determine the composition of water through burning hydrogen in oxygen, described in his Traité Élémentaire de Chimie published in 1789. Before the reaction, the air inside the apparatus was removed by a vacuum pump. Then oxygen was let in through the right pipe and hydrogen through the left pipe. As soon as hydrogen entered the contrainer, an electric spark was initiated to ignite the combustion. The flux ration of oxygen and hydrogen was 1:2 and combustion was maintained for a long time. The water generated was collected in the bottom of the container. After the experiment, a crucial check was that the total mass of oxygen and hydrogen consumed should equal the water produced. Through this experiment, Lavoisier proved that water was not an element, but a compound made of hydrogen and oxygen, with a weight percentage of 15% and 85%, respectively. We now know that the correct percentage should be 11% for hydrogen and 89% for oxygen. In terms of accuracy, Lavoisier’s experiment was not as good as Cavendish’s.
Above is Lavoisier’s apparatus for studying the reaction between water and iron at high temperature, described in his Traité Élémentaire de Chimie published in 1789. The goal of this experiment was to determine the composition of water. Through heating the distilled water inside the right retort, water steam was transported to the high-temperature glass tube, reacting with the spiral shaped ion sheet inside. The reaction between water and iron generated black iron oxide (Fe3O4) and hydrogen. The unreacted water steam passing the long tube was condensed in the condenser and collected in a bottle. Hydrogen was collected with the bell jar sitting on the platform of the water trough and its mass measured. The mass increase of the iron sheet came from the oxygen in water. Lavoisier carefully checked that the mass of reacted water (the mass loss of the retort subtracted from the mass increase of the collecting bottle) equaled the total mass of hydrogen collected in the bell jar and oxygen calculated from iron weight increase. Through this complicated setup, Lavoisier again found the hydrogen and oxygen weight percentage of water is 15% and 85%, respectively. This result was again not as accurate as Cavendish’s.
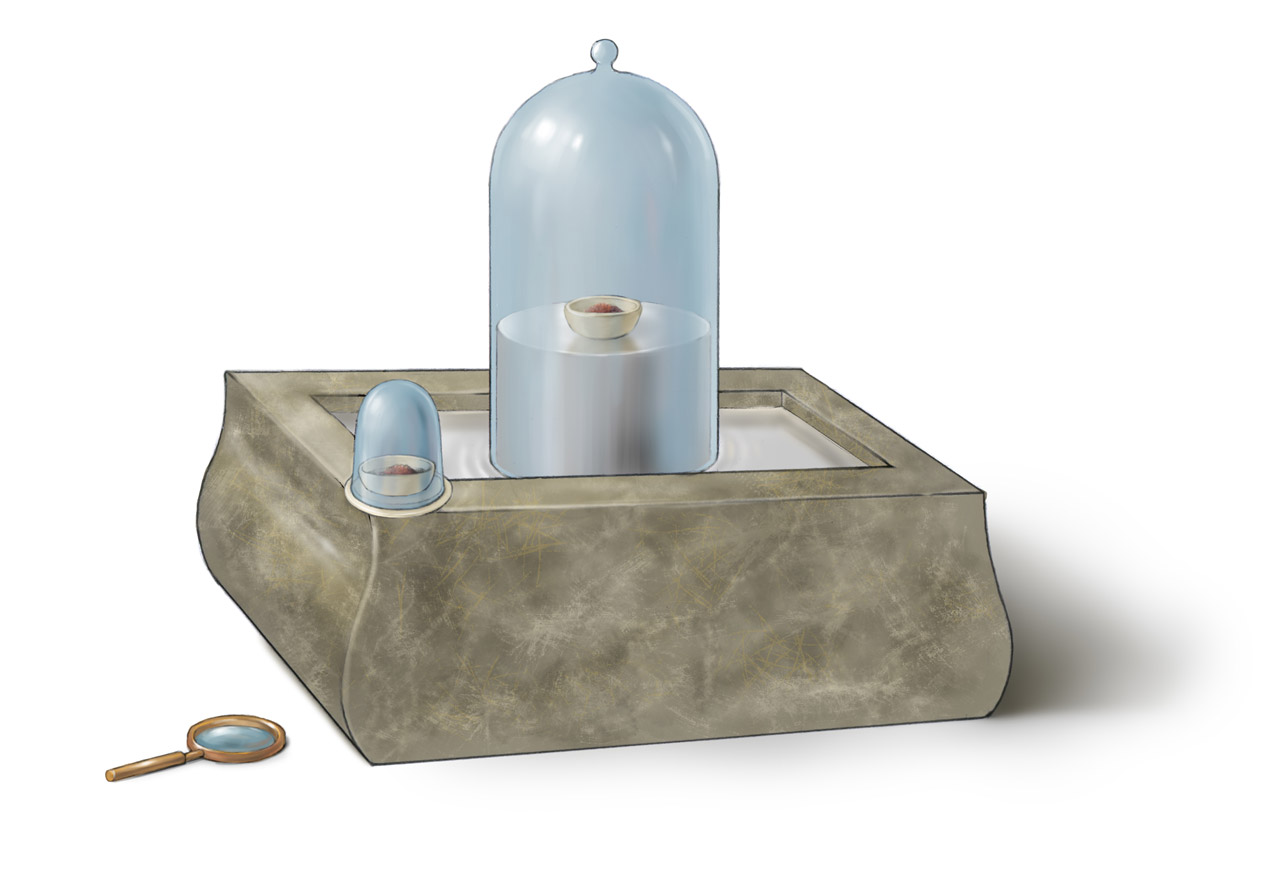
Above is Lavoisier’s apparatus for studying red phosphorus combustion inside oxygen, described in his Traité Élémentaire de Chimie published in 1789 (a photorealistic CG reconstruction can be found here. The bell jar over the mercury reservoir contained oxygen. The red phosphorus was transferred into the bell jar through a small sealed container. The red phosphorus was ignited by a red-hot ion stick with a special shape or a burning glass. Lavoisier also used this apparatus to studied the combustion of other substances. In his book, he warned his readers not to use the apparatus to study volatile substance like alcohol, which was likely to explode. With this apparatus, Lavoisier could determine the amount of oxygen reacted with phosphorus. However, he could not determine the amount of phosphorus oxide produced in the reaction. As a result, he designed a new apparatus to make more accurate measurement.
Above is Lavoisier’s another apparatus for studying red phosphorus combustion inside oxygen, described in his Traité Élémentaire de Chimie published in 1789. First he placed a small bow containing red phosphorus inside a glass globe, then sealed the opening. After the lute dried up, the whole apparatus was weighted. Then the air inside the globe was removed by a vacuum pump. Another through another tube, oxygen was introduced. A burning glass was used to ignite the red phosphorus and oxygen flow was maintained until all the phosphorus was burnt up. By weighting the whole apparatus at the end, the weight of the phosphorus oxide could be determined.
Other Chemists