Carbon Nanostructure
Generating Research Excitement Again and Again
Scientists have realized two allotropes of carbon two hundred years ago: the hard and transparent diamond and the soft and black graphite. In the early 20th century, scientist identified the carbon atom arrangement in diamond and graphite with X-Ray diffraction, and thus provided the structural explanation of the difference between them. The discovery of C60 and other fullerenes is a prologue of many nanocarbon materials. Afterwards, the discovery of carbon nanotubes (in 1992) and the successful exfoliation of graphene (in 2004) have generate great excitement among scientists.
Fullerenes, carbon nanotubes, and graphene have very unique properties. For instance, C60 molecule can absorb most of solar spectrum. Because of its semiconductor property, C60 can be used in low-cost organic solar cells. Carbon nanotubes can be classified into semiconducting or metallic according to their atomic structure. Carbon nanotubes also have great mechanic properties and are the strongest one-dimensional materials. Graphene has become a stellar nanomaterial due to the extremely high tensile strength, electrical conductivity, transparency, and being the thinnest two-dimensional material in the world.
Carbon-based nanomaterials have been applied in many fields. Besides the above-mentioned C60-based organic solar cells, carbon nanotubes can be used to improve the mechanical property of polymers or metals. But there seems to be some hypes on carbon nanomaterials. Many researchers and companies predicted that carbon nanotubes would be the next generation material in transistors to replace silicon after its discovery. Nevertheless, after 30-year research, this goal has not been achieved. The main reason is the difficulty to integrate such a large amount of carbon nanotubes at the microscopic scale. After its emergence, graphene was rapidly regarded as a material that will change the world by many news media. Scientists also forecasted that the research of graphene would result in high-performance computer processors, flexible and transparent displays, and high capacity batteries. But the large-scale applications of graphene have not yet appeared. It is perhaps too early to conclude on the prospect of graphene and other types of carbon nanomaterials. After all, it has taken more than one hundred years for silicon to be used in widely in transistors. We need to wait and see whether the carbon nanomaterials will change the world one day.
Top diagram: a C60 molecule.
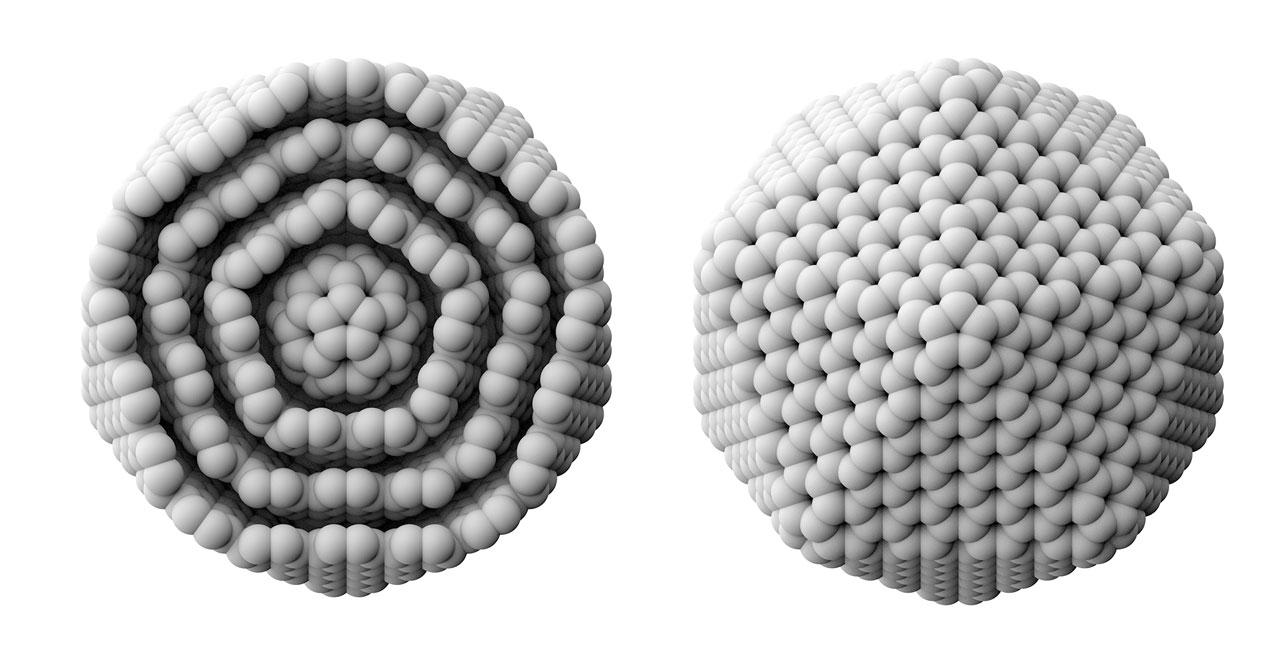
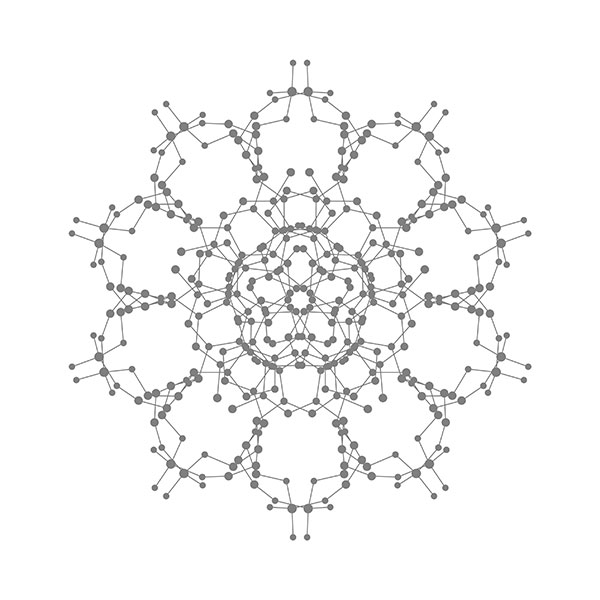
Right: Cross section of a carbon onion (C60@C240@C540@C960), left: C960.
Note: all number should be subscripts.
Fullerenes. In 1985, Curl, Kroto, and Smalley discovered C60 for the first time when they use laser to vaporize graphite. Because the common structure with the spherical roof designed by Fuller, they named this molecule as fullerene. The large-scale synthesis of fullerene after 1990 generated the great interests for scientists. The diameter of a C60 molecule is around 1 nm, suggesting a representative nanomaterial in the field of nanoscience. The above three scientists were awarded Nobel Prize in chemistry in 1996. C60 is actually the smallest one in the fullerene family. In 1992, D. Ugarte discovered a giant fullerene with a multi-layered carbon onion structure. The above image illustrates a carbon onion in which the center sits a C60 and outer layers correspond to C240, C540, and C960 in turn. [Figure reference: Wang B.-C. et al. Synthetic Met. 55-57, 2949 (1993)]
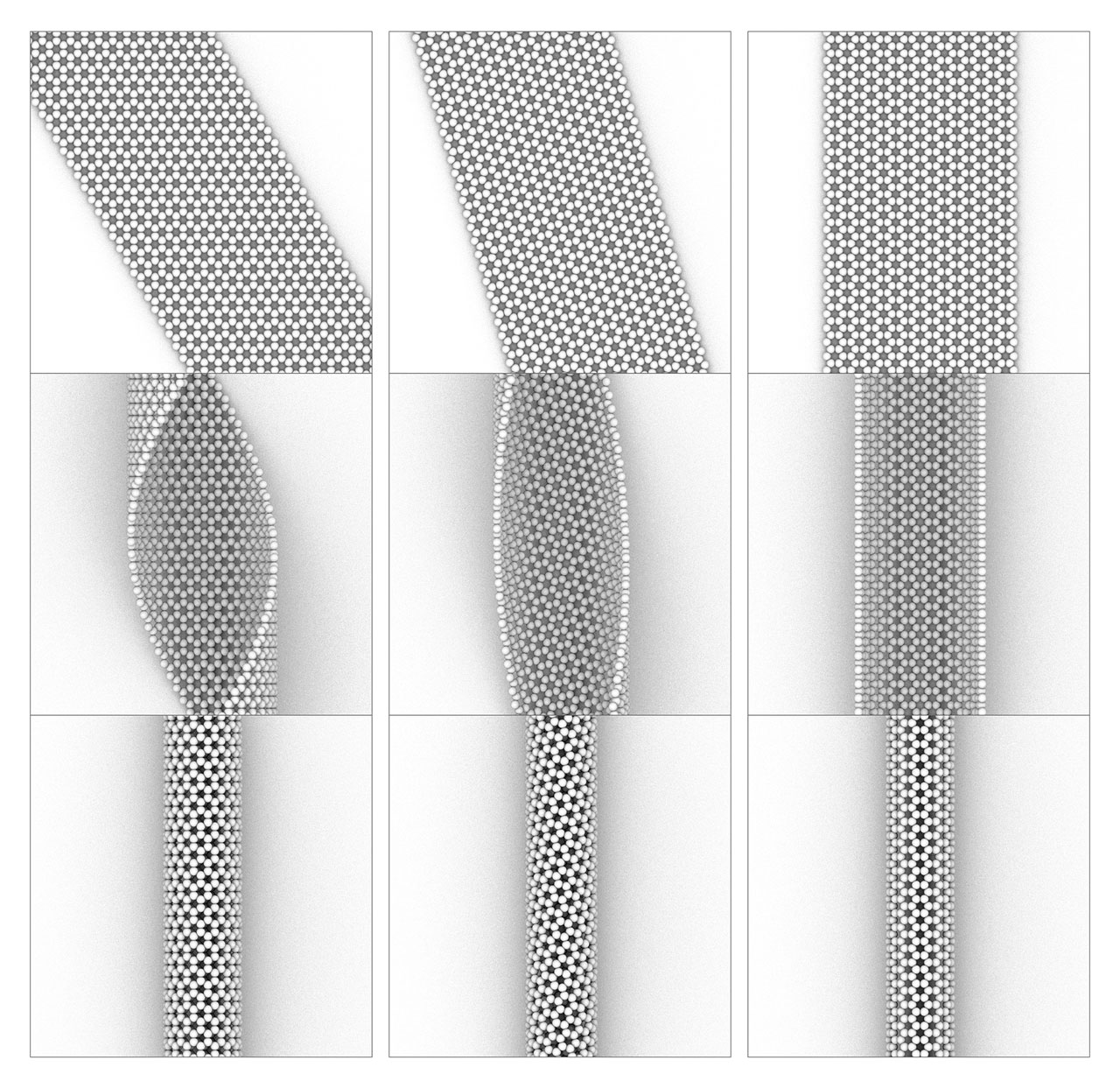
Left column: zigzag carbon nanotube (16, 0), middle column: chiral carbon nanotube (12, 4), right column: armchair carbon nanotube (8, 8).
Carbon nanotubes and graphene. C60 is only the inception of a wave of research in carbon nanomaterials. The carbon nanotube reported by Sumio Iijima in 1992 and single layer graphene prepared by a simple mechanical exfoliation method by Geim and Novoselov triggered tremendous interests for scientists. Carbon nanotube and graphene possess unparalleled properties to any other materials. For example, carbon nanotube is the strongest one-dimensional material, and graphene is the thinnest transparent conductor. Scientists still show increasing enthusiasm on these materials. Geim and Novoselov won the Nobel Prize in physics in 2010. Carbon nanotube is structurally related to graphene. Different carbon nanotube can be derived from different manners of roll-up of a graphene nano-ribbon, as shown in the left figure. [Figure reference: White C. T. et al. Phys. Rev. B 47, 5485 (1993)]
Other Topics