Chemical Symbols
The Essential Part of Chemical Language
Chemical nomenclature and symbols are two of the most important parts of the language of chemistry. Early nomenclature was completely empirical. As result, the confusion arose between substance names and their chemical properties. For example, “oil of vitriol” was the name of sulfuric acid, which is not an oil at all. By the mid-18th century, a large number of new substances were discovered thanks to the rapid development of chemistry. The old nomenclature could no longer meet the needs of chemist. Finally, at the end of 18th century, Antoine Lavoisier started chemical revolution, replacing the old empirical nomenclature with a new one based on chemical composition and properties.
The earliest use of chemical symbols could be traced back to ancient Egypt glyphs and Greek manuscripts. Experts think that a small number of chemical symbols could be evolved from Egypt glyphs; a few others came from Greek manuscripts. The majority symbols used by alchemists from the 17th to 18th century were created by the alchemists themselves. Some of the alchemy symbols were pictorial representations of the chemical apparatuses, while others were just random graphical constructions. The main purpose of using symbols was to reduce texts and improve the reading speed of alchemy books. However, many alchemists believed that the secrets of philosopher’s stone were hidden within the symbols and worked endlessly to decipher them. Also, alchemy symbols could also cause confusion: some symbols with different meanings looked very similar, and same symbols might be used to indicate different meanings by different alchemists. Because of the mystical and confusing nature of alchemy symbols, some scholars created new symbol systems to replace them. Lavoisier proposed that chemical information should also be encoded in symbols. In 1808, John Dalton published a new symbol system which was an important achievement. Dalton designed a circular symbol for each element known in his time. In his system, a compound was a graphic assembly of the element symbols based on their proportion. However, Dalton’s symbols were not easy to write and remember, just like old alchemy symbols. We should thank Jacob Berzelius for the chemical symbol system we are using today (e.g. Na stands for sodium, NaCl for salt, and H2O for water). Although Berzelius was not the first person who used the initial letters of element names as symbols, he was the first to apply this system to all the chemical substances known at his time.
Top diagram: the Sun symbol representing gold during Alchemy era.
Top left: fire, top right: water, center: either, bottom left: air, bottom right: earth.
Kepler’s fifth element. The well-known concept of modern elements was proposed by Antoine Lavoisier at the end of 18th century. Prior to this, the four-element theory, including “fire”, “water”, “air”, and “earth”, or the five-element theory with “ether” as the fifth element dominated the outlook of the world among western scholars. In “Harmonice Mundi” published in 1619, Kepler associated the five elements with five platonic polyhedra. According to Kepler’s point, as the most penetrating element, “fire” should correspond to tetrahedra because it is the sharpest in platonic polyhedra. “Ether” is different to all other elements. Because it doesn’t have essences such as “cold”, “hot”, “dry”, or “wet”, it should be related to icosahedra which is the closest to spheres. In the above figure, icons next to illustrations are the corresponding symbols. [Figure reference: Kepler, J. Harmonice Mundi (1619)]
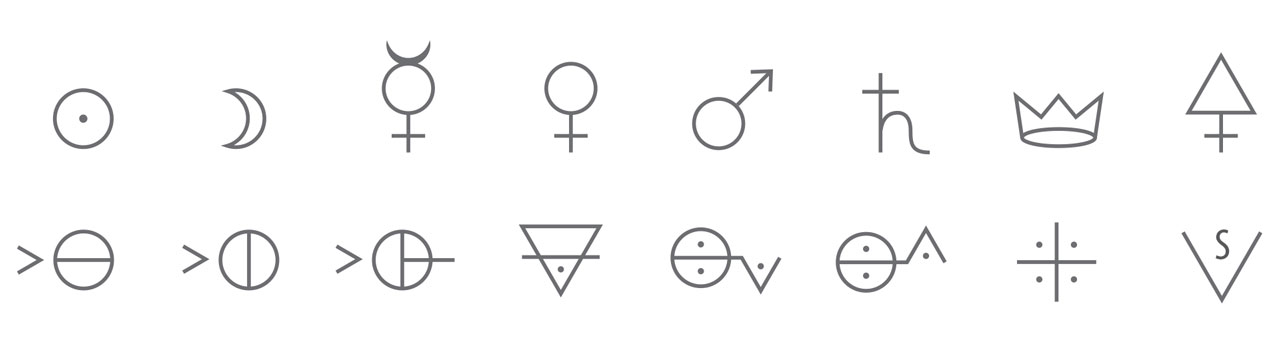
First row from left to right: gold (Au), silver (Ag), mercury (Hg), copper (Cu), iron (Fe), lead (Pb), antimony (Sb), sulfur (s).
Second row from left to right: hydrochloride acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), calcium carbonate (CaCO3), potassium carbonate (K2CO3), Ammonia solution (NH4OH), acetic acid (CH3COOH), alcohol (CH3CH2OH).
Note: all number should be subscripts.
Alchemy symbols. The above figure is the part of alchemy symbols published in “table of affinities” by Geoffroy in 1718. Similar symbols were widely adopted in the alchemy era after Renaissance. Geoffroy’s “table of affinities” prompted the popularity of alchemy symbols. Geoffroy believed that those symbols in the table could make a clear relationship of chemical reactions between each substance. However, more and more scholars rejected these symbols due to their shortcomings of mysticism, difficulty to remember, and confusing. In 1813, Berzelius upgraded the chemical symbol system. (The only difference between his system and modern one is that he used superscripts instead of subscripts to represent the proportion of elements.) In contrast to alchemy symbols, modern chemical symbols are informative and much easier to write and remember, especially when it comes to represent the chemical composition and molecular structure of organics. [Figure reference: Geoffroy, É. F. Mémoirés de l'Academie Royale des Sciences (1718)].
Other Topics