Organic Molecular Structure
Countless Possibilities of Carbon Atoms
In the initial state of organic chemistry, it is generally believed that the chemical property of an organic compound is only determined by its chemical composition. However, scientists gradually discovered some organic compounds that possessed the same chemical composition but exhibited different chemical properties. Such discovery strongly indicates that the combination and the arrangement of atoms inside the molecules were of great influence on the properties of organic compounds. In 1830, Berzelius first used the name of “isomers” for this type of organic compound.
The improved accuracy of elemental analysis and further research on chemical properties led to the discovery of common functional groups with fixed chemical compositions, such as methyl (CH3-) and benzyl (C6H5-). In 1858, Friedrich August Kekulé proposed a theory of “tetravalency of carbon”. According to his theory, a carbon atom should link to other atoms (including other carbon atoms) by 4 chemical bonds. This was one of the most important theories for understanding the structure of organic molecules.
In 1875, Jacobus Henricus van’t Hoff proposed the tetrahedral model of tetravalent carbon atoms and applied this model to explain enantiomers that are mirror images of each other with opposite optical activity. From then on, the importance of determining 3D structure of organic molecules was widely accepted and stereochemistry became a new branch of chemistry.
In the 20th century the X-Ray diffraction technology became a powerful tool for chemists to analyze 3D molecular structures. A series of structural challenges were finally resolved perfectly, such as the verification of the planar structure of benzene rings and the absolute structural confirmation of enantiomers. To this end, scientists were capable of solving the complex structure of natural products, which was useful for their chemical synthesis and the study of their biological activities. In this regard, an important achievement was solving the 3D structure of Vitamin B12 that contains a total of 181 atoms by Dorothy Crowfoot Hodgkin and her team in 1955.
Top diagram: electron density distribution of a benzene molecule. Ref.: Cox, E. G. Rev. Modern Phys. 30, 159 (1958).
First row: Kekulé structure (1861).
Second row: Loschmidt structure (1861).
Third row: Brown structure (1868)
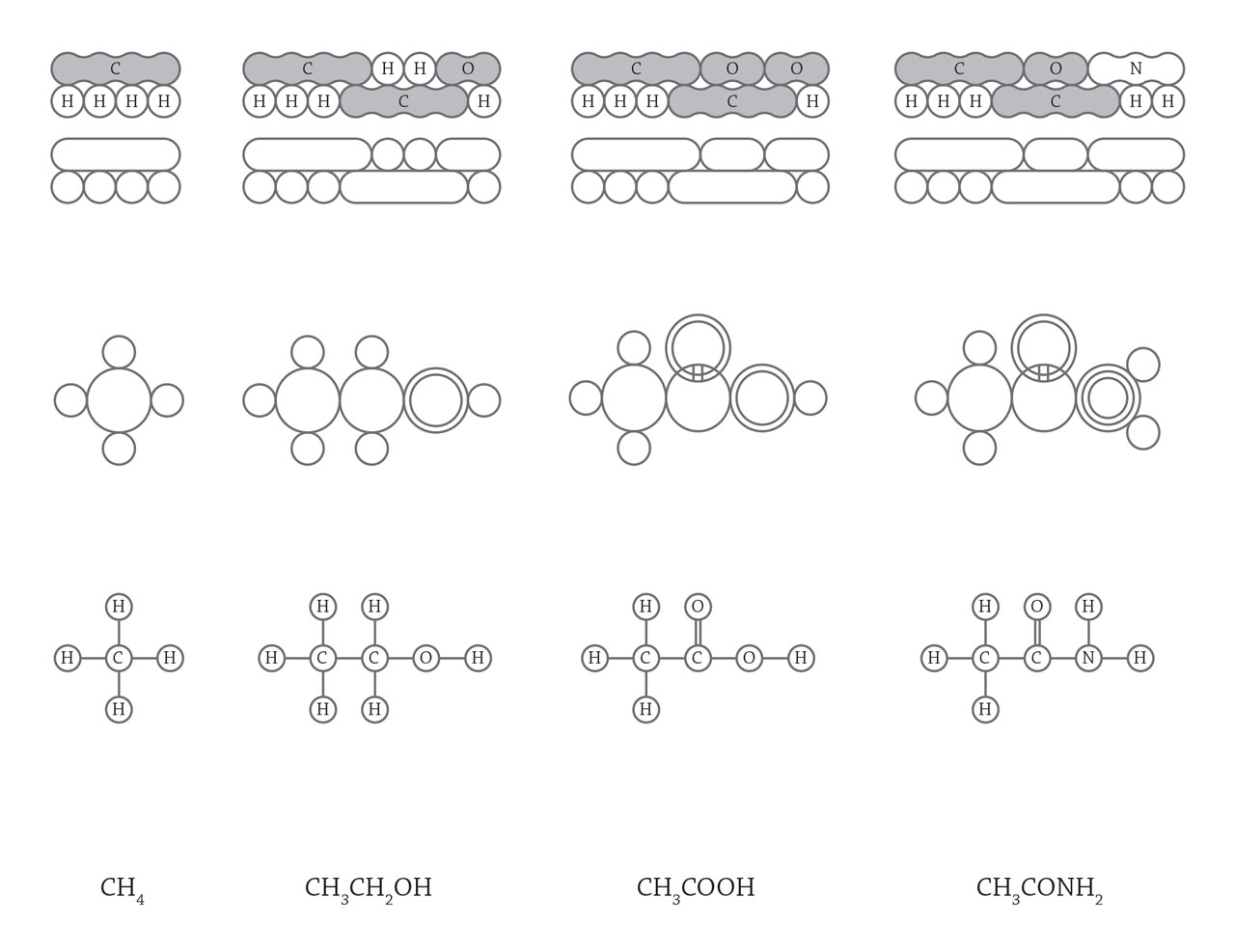
Organic molecular structure. In 1817, scientists started to realize the existence of isomers, molecules with the same chemical composition but exhibited different properties. It indicated that the arrangement of atoms inside a molecule could influence its properties. With a deeper understanding to the structure of organic molecules, scientists applied structural formula to represent the arrangement of atoms. The above figure shows some structural formulas used from 1861 to 1868. As the structure of methyl, carboxyl, and hydroxyl groups are generally accepted, the much concise organic structural formula are used today by directly using function groups like CH3, OH, and COOH (the last line in the above image). [Figure reference: Ihde, A. J. The Development of Modern Chemistry (1964)]
First row from left to right: first two are Kekulé benzene structures proposed in 1865, the last was Kekulé benzene structures proposed in 1872.
Second row from left to right: Clauss structure I, Clauss structure II, Ladenberg Structure I, Landenberg structure II, Armstrong structure.
The structure of benzene. Scientists determined that the chemical formula of benzene was C6H6 in the 19th century. However, the lack of carbon-carbon double bond properties has confused those scientists. In 1865, Kekulé proposed a hexagonal ring with alternative single bonds and double bonds. However, a number of chemists did not agree his benzene structure and proposed other structures (the low part in the above figure). To overcome the limit of his early model, Kekulé gave a new model, in which he suggested that single bonds and doubles rapidly switched inside the benzene. This model could explain why the properties of benzene are different to those molecules with double carbon-carbon bonds. Although Kekule’s model had its limitations, it was still used by chemists until now. [Figure reference: Ihde, A. J. The Development of Modern Chemistry (1964)]
Most of scientists believed that benzene was a planar molecule while others thought that it was a buckled structure with three carbon atoms above the molecular plane and other three atoms below the plane. It was not until K. Lonsdale’s X-ray diffraction experiment on the crystal structure of hexamethylbenzene (hexamethylbenzene is solid while benzene is liquid at room temperature) did people finally accept the planar structure of benzene. The carbon-carbon bond length in benzene was also determined as 1.4 Å, sitting between single bonds and double bonds.
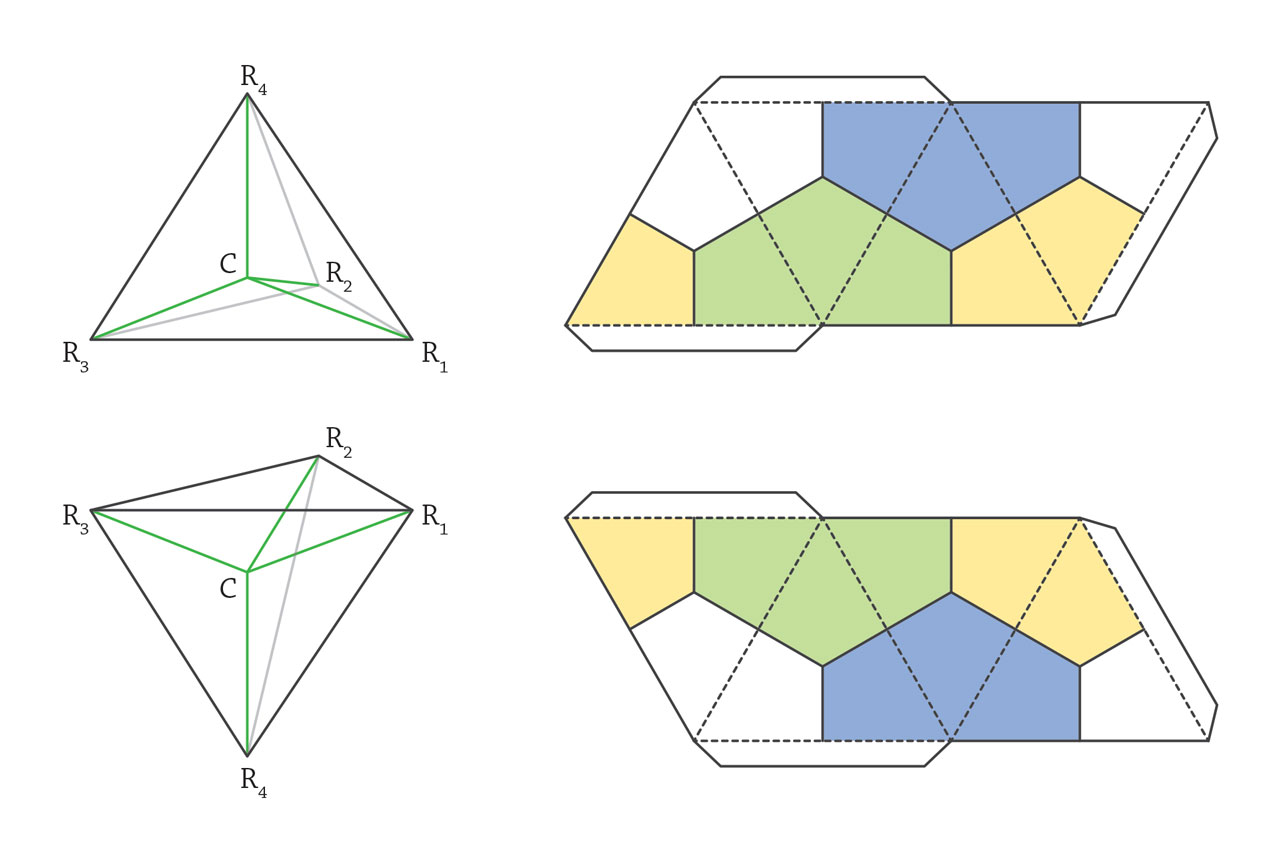
Stereochemistry. In early 19th century, J. B. Biot discovered optical activity of solutions of certain organic compounds. The polarization of linearly polarized light rotated when it passed through such solutions. Pasteur systematically investigated this phenomenon and discovered two types of tartaric acid molecules with identical chemical properties but opposite optical activities. He defines these two types of tartaric acid as L- and D-tartaric acid, respectively, and postulated that it should relate to a certain type of structural asymmetry inside the molecules. In 1857, van’t Hoff pointed out that the optical activity should originate from the chiral carbon atom and claimed the carbon-centered tetrahedral model. When the central carbon atom bonds to four different atoms or group on the vertices, enantiomers, the mirror image of each other, should be raised. To have his theory be accepted, van’t Hoff published “Die Lagerung Der Atome Im Raume” in which instructions were provided to make two tetrahedra, mirror image of each other. Although van’t Hoff’s proposal caused significant arguments, the chiral atom finally became one of the most important concepts in stereochemistry. The chemists still could not determine the absolute structure of enantiomers. This puzzle was not solved until the X-ray structural analysis of natural D-tartaric acid. It’s such a relieve for chemists, as the early guessed D- structure, which has 50% chance of being the correct one, was consistent with the results from diffraction. [Figure reference: van’t Hoff, J. H. La chimie dans l’espace (1875); van’t Hoff, J. H. Die Lagerung Der Atome Im Raume (1877)]
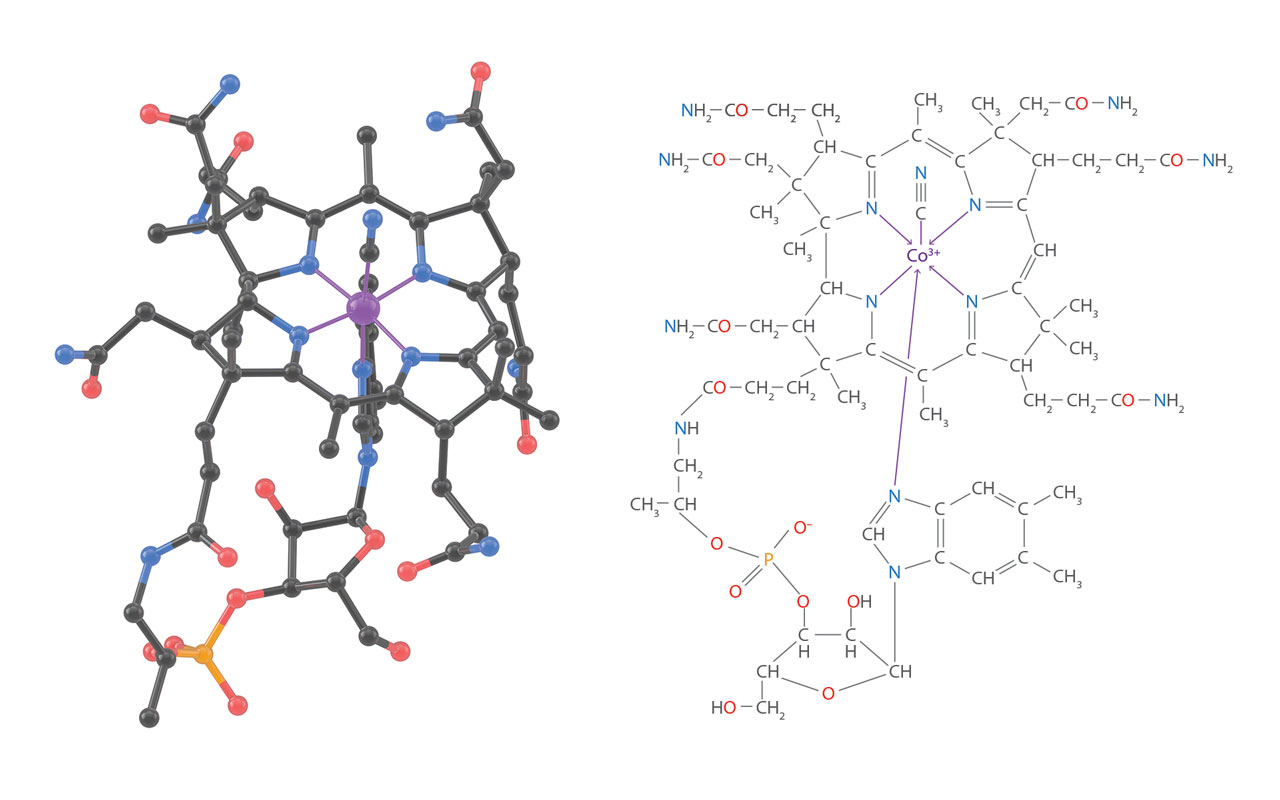
3D structure of Vitamin B12. In 1955, Hodgkin and her colleague published the three-dimension structure of Vitamin B12 solved by X-ray diffractions (up left figure, H atoms are not shown). This was a milestone in the history of organic chemistry. Hodgkin was awarded Nobel Price in chemistry in 1964. Discovered in the 1940s’, Vitamin B12 is a very complex organic molecule with 181 atoms (molecular formula, C63H88CoN14O14P). A cobalt atom sits at its center. The total synthesis of B12, another milestone in history, was achieved by a long-term cooperation between American and Swiss scientists for 17 years. [Figure reference: Hodgkin, D. C. et al. Nature 178, 64 (1956); Marino, N. et al. Inorg. Chem. 50, 220 (2011)]
Other Topics