Chemical Bonds
The Foundation of All Chemical Structures
Chemical bond is a very important concept in chemistry. Atoms join together via covalent bonds to form molecules. Positive and negative ions form ionic crystals through ionic bonds, and metal atoms form crystals by metallic bonds. All chemical bonds involve electrons. However, before the discovery of the electron, there were a few primitive theories of chemical bonds. For example, In Opticks, Newton wrote “particles attract one another by some forces, which, in immediate contact is exceedingly strong, at small distances performs the chemical operations above mentioned, and reaches not far from the particles with any sensible effect.”
After Bohr’s quantum atomic model, the most influential chemical bond theory is the one based on the octet rule proposed by Lewis in 1916. Using the octet rule, Lewis successfully explained the formation of ionic bonds inside ionic crystals. In addition, he introduced the concept of electron pair and covalent bond. Even today, we are still using electron pairs and Lewis structure to teach the basic concepts of chemical bonds. In addition, valence shell electron pair repulsion (VSEPR) theory developed based on Lewis theory can be used to intuitively predict the 3D molecular structure of simple compounds.
The establishment of quantum mechanics accelerated the development of chemical bond theories. Valence bond theory, molecular orbital theory, hybrid orbital theory, and density functional theory are important theoretical methods to study chemical bonds based on quantum mechanics. As computers are becoming more and more powerful, software applications based on these theories are used routinely by chemists to study molecular structures. For example, chemists could use computer to simulate the breaking and formation of chemical bonds, providing theoretical explanation and reference to experiments.
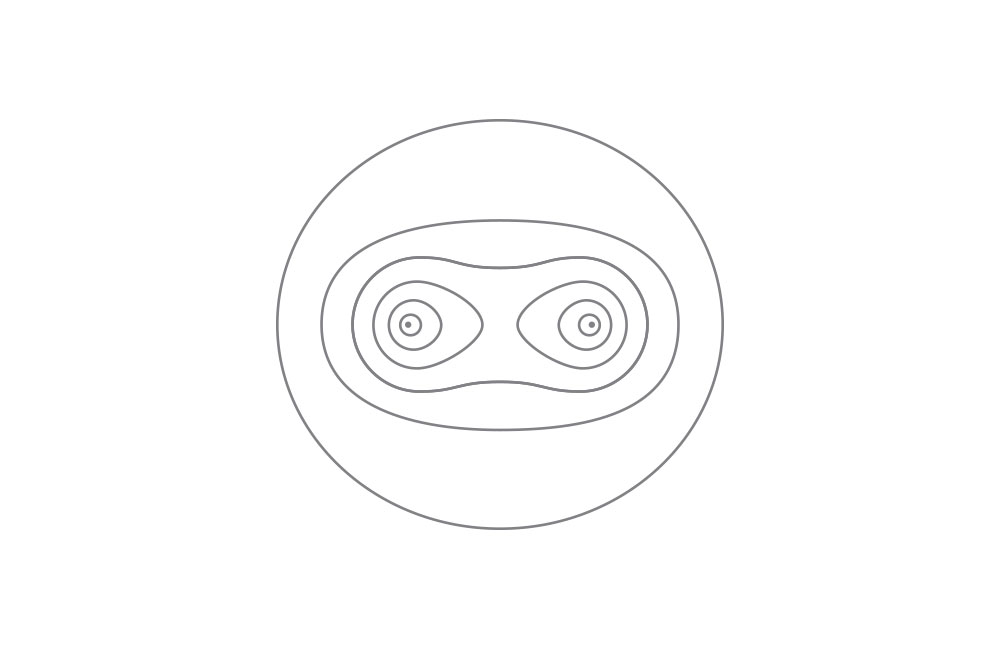
Top diagram: electron distribution inside a hydrogen molecule. Ref.: London, F. Zeitschrift für Physik 46, 455 (1928).
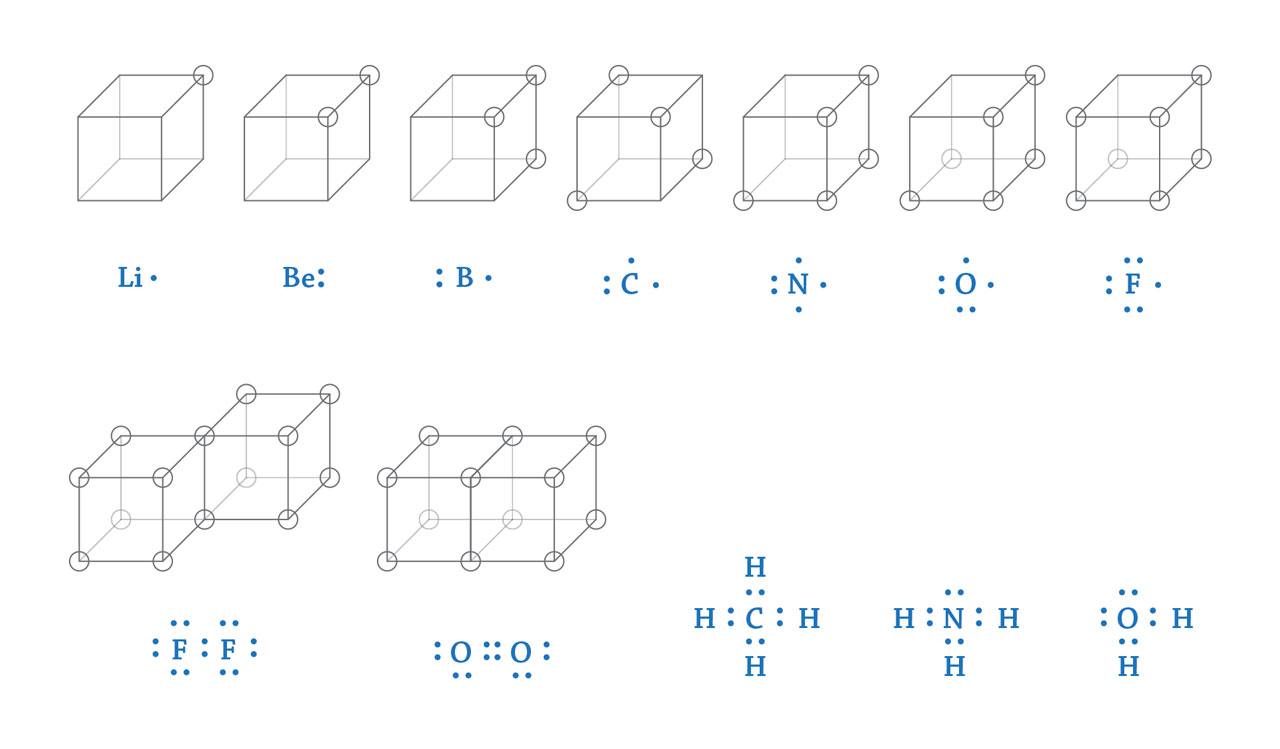
Top row from left to right: lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine.
Bottom row from left to right: fluorine molecule, oxygen molecule, methane molecule, ammonia molecule, and water molecule.
Lewis’s chemical bonding theory. In 1916, Lewis proposed a chemical bonding theory based on Bohr’s atomic model. In order to facilitate understanding, he placed all outmost shell electrons on each vertex of a cube. A stable atomic structure is obtained when all vertices are occupied or unoccupied. A Li atom with only one electron on the outmost shell prefers to donate it to a F atom that has seven electrons on the outmost shell. Then the Li atom become Li+ ion while F atom changes to F- ion with completely filled outmost shell. These two types of ions form a stable compound, LiF. Similarly, an O atom with 6 outmost shell electrons need to combine with two Li atoms, each of which should transfer one electron to the O atom and form the ionic compound of Li2O. In covalent compounds, atoms will share one edge of the cube, e.g. two F atoms forming a single bond, or share one face of the cube, e.g. two O atoms forming a double bond. By doing this, all atoms reach its stable configuration. In one of his papers published in 1916, Lewis also gave a representation of electron configuration that used black dots surround element symbols to manifest the outmost shell electrons (blue dots in the above figure). This configuration was called “Lewis structure”, which has been widely used as an effective tool to explain chemical bonding in many chemistry textbooks. [Figure reference: Lewis, G. N. J. Am. Chem. Soc. 38, 762 (1916)]
Top row from left to right: AB2, AB3, AB4.
Bottom row from left to right: AB5, AB6, AB6.
Valence-shell electron-pair repulsion (VSEPR) model. Based on Lewis’s chemical bonding theory, Nevil Sidgwick et al. developed a valence-shell electron-pair repulsion theory, which is able to predict the 3D structure of simple molecules by considering the repulsion of electron pairs. For instance, the repulsion among four electron pairs inside methane molecules results in the most stable tetrahedral structure. The carbon atom sits at the center of the tetrahedron while four hydrogen atoms are at four vertices. The above image includes some of the simple three-dimensional structures according to the VSEPR theory. [Figure reference: Sidgwick, N. V. and Powell, H. M. Proc. R. Soc. Lond. A 176, 153 (1940)]
Other Topics