Atomic Structure
From Atomic Theory to Quantum Mechanics
The atomic theory has been around for a long time. However, from its inception by ancient Greek philosophers in the 5th century BC to its revival by scientists such as Boyle and Newton during the Renaissance, atomic theory seemed to be more relevant to philosophy and physics but not very useful to explain chemical properties of matter.
In the early 19th century, Lavoisier’s theory of chemical elements and Proust’s law of constant proportion of chemical compounds set a foundation for Dalton’s atomic theory. In 1803, Dalton described his theory clearly in his notebook: 1) Chemical elements are composed of very small, indivisible atoms. 2) All atoms of an element are the same, atoms of different elements are different, and the difference is in the weight of atom. 3) The ratios of different atoms in compounds are simple numerical ratios, such 1 : 2 or 2 : 3. Dalton’s theory provided a clear microscopic explanation to Proust’s law of constant proportion, and it was quickly accepted by chemists. Later with the development of analytical chemistry, all atomic weight and chemical formula of compounds were determined.
The discovery of the electron at the end of the 19th century invalidated the indivisibility of Dalton’s atom. In the early 20th century, many models of the internal atomic structure were proposed. In 1913, Bohr presented a revolutionary quantum atom based on Rutherford’s nucleus model and Planck’s quantum theory. With Bohr’s model, the emission spectrum of atomic hydrogen could be beautifully explained with very simple mathematical equations. Bohr’s model was a triumph of quantum theory in the early 20th century. Later, the establishment of quantum mechanics completely changed our understanding of the atomic world. Today scientists are continuing the research on the atomic structure. Nuclear physicists are working on problems such as how protons and neutrons arrange inside a nucleus and what is the ultimate size of a nucleus. We now know that protons and neutrons are made of even smaller basic particles (quarks). By conducting high-energy experiments inside magnificent particle accelerators, particle physicists are now searching for the basic particles of matter and radiation (such as visible light), to answer the ultimate question: where does matter come from?
Top diagram: De Broglie's wave.
Top row from left to right: hydrogen (H), carbon (C), nitrogen (N), oxygen (O), phosphorous (P), sulfur (S).
Middle row from left to right: iron (Fe), zinc (Zn), copper (Cu), lead (Pb), Silver (Ag), platinum (Pt), gold (Au), mercury (Hg).
Bottom row from left to right: carbon monoxide (CO), carbon dioxide (CO2), nitric oxide (NO), nitrous oxide (N2O).
Note: all number should be subscripts.
Dalton’s atomic theory. Greek philosophers first introduced a primitive atomic theory in the 5th century BCE. After Renaissance, Scientists such as Boyle and Hooke picked up atomic theory or p-what again and used it to explain natural phenomena. Dalton first detailed his atomic theory in 1803. Two major differences between his theory and the previous ones are: 1) different elements are composed of different atoms, 2) different atoms have different weight. In the early 19th century, Dalton’s theory was widely accepted by chemists, advancing the development of chemistry at the theoretical level. In his book A New System of Chemical Philosophy published in 1808, Dalton designed a circular symbol for each element known in his time and used combination of these symbols to represent compounds (above). Because it was difficult to determine accurate atomic weights, Dalton’s atom ratios of many compounds were wrong. Here we only show a few of his compound representations with correct atom ratios. [Figure reference: Dalton, J. New System of Chemical Philosophy (1808)].
Top left: dynamid model, top right: Saturn model, bottom left: plum pudding model, bottom right: nucleus model.
Early models of atomic structure. Dalton’s atoms are indivisible hard spheres. Since Thomson discovered the electron in 1897, however, new experiment evidence made scientists realize that atoms might have sophisticated inner structures. In the early 20th century, many atomic models emerged. Four of them are presented in the above figure, with red and blue colors representing positive and negative charges, respectively. Dynamid Model, proposed by Lenard in 1903, the atom is an empty shell with dynamids in the center. A dynamid is made of a single positive charge and a single negative charge connected together. Saturn Model, proposed by Hantaro Nagooka in 1904, the atom is similar as Saturn, with electrons orbiting a positively charged sphere in the center. Plum Pudding Model, proposed by J. J. Thomson in 1904, the atom is composed of a positively charged shell and electrons imbedded in the shell. In Nucleus Model, proposed by Rutherford in 1911, the atom consists a positively charged nucleus in the center and electrons moving around it. The nucleus is very small but contains nearly all of the mass of the atom. [Figure reference: Ihde, A. J. The Development of Modern Chemistry (1964)]
The center dot is the nucleus.
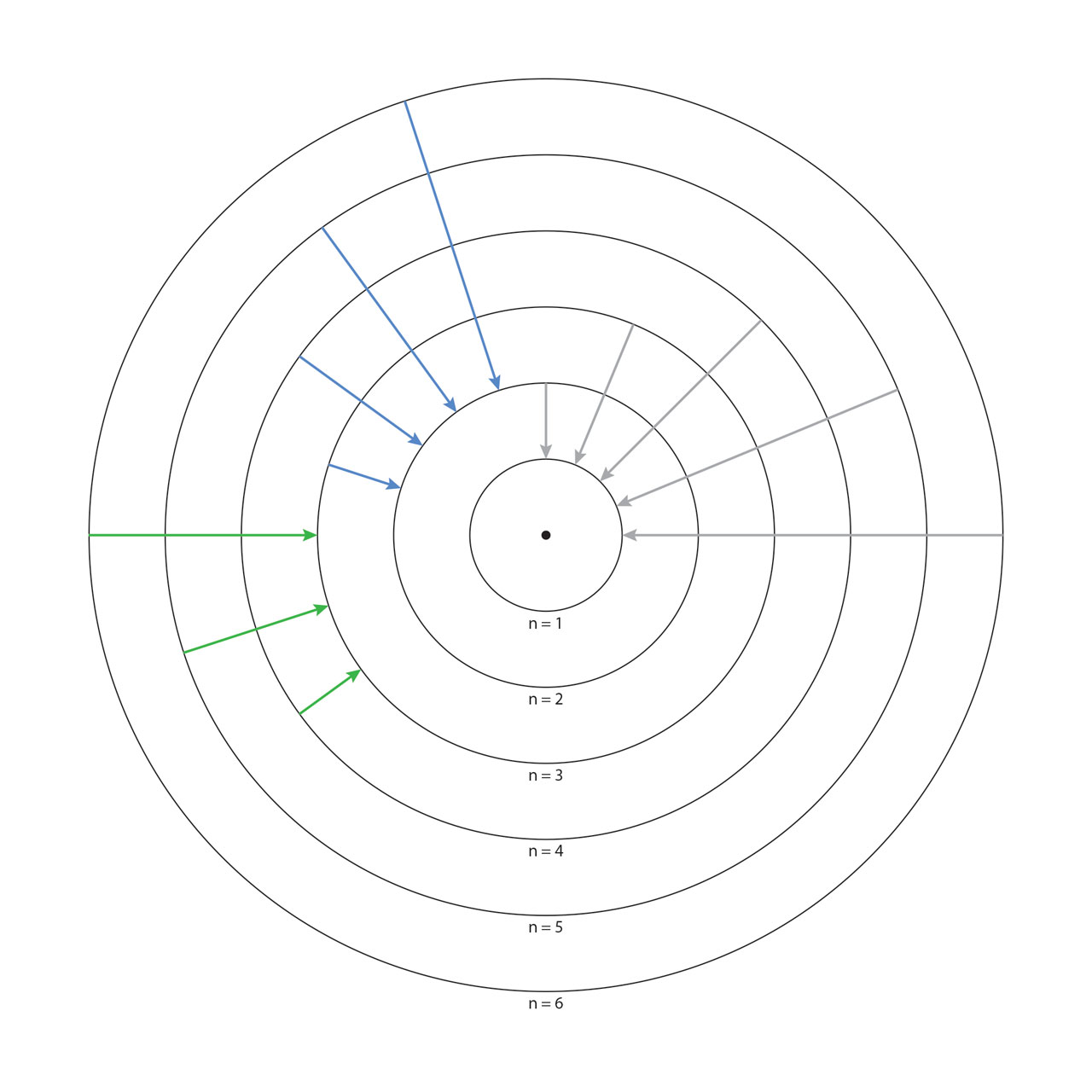
Bohr’s quantum atom. In 1913, Bohr proposed a new quantum atomic model, which was one of the most revolutionary theoretical models in the history of science. Bohr’s quantum atom is similar to a microscopic solar system, with a series of electron orbitals circulating the positively charged nucleus in the center. Every orbital is characterized by a specific energy, with lower-energy orbitals closer to the nucleus and higher-energy orbitals further away from the nucleus. An electron must belong to a certain orbital, with its energy the same as the orbital’s. An electron can transfer from one orbital to another only if it absorbs (for low-energy orbital to high-energy one) or emits (from high-energy orbital to low-energy one) a photon and the photon’s energy is the same as the energy difference of the two orbitals. Bohr’s model provided perfect explanation to the hydrogen atomic spectrum (above we showed the corresponding electron transition of Lyman, Balmer and Paschen series of hydrogen spectrum), and set a foundation for Lewis’ chemical bond theory. In 1922, Bohr was awarded the Nobel Prize in physics for his contribution in quantum atomic model. [Figure reference: Bohr, N. Phil. Mag. 26, 1 (1913)]
Top row from left to right: 2p, 3d, and 3d orbitals.
Bottom row: all three are 4f orbitals.
Atomic orbitals of quantum mechanics. Although Bohr’s quantum model successfully explained some properties of hydrogen atoms and other single electron ion such as He ion, it was very limited for multi-electron atoms. From 1920 to 1930, de Broglie, W. Heisenberg, I. Schrodinger, and other physicists established quantum mechanics, which became the fundamental theory for understanding the atomic and molecular world. In quantum mechanics, an atomic orbital is interpreted as the possibility of finding an electron around a nucleus, which can be calculated using the elegant Schrodinger equation. To make these abstract mathematical concepts more intuitive, chemists usually use graphics to represent atomic orbitals. For the orbitals above, the 3D surfaces are selected based on isosurfaces, inside which the possibility of electron occurrence is 90%. (Figure reference: the three-dimensional atomic orbit models by Dr. S. Immel from Darmstadt Applied Science and Technology University)
Other Topics